“Democracy is the worst form of government, except for all the others…” – Winston Churchill
In this Procyon Perspectives, we cover several topics. We will provide a brief overview of October markets, comment on the US elections, take a deeper dive into the world of vaccines, and finally finish up with a few year-end financial planning ideas.
October Markets
Financial markets sputtered in October as the news was dominated by two main topics: the resurgence of Covid-19 and the US elections.
The S&P 500 finished October -2.66% with small and mid-cap stocks doing better (Russell 2500) up +1.81%. Value outperformed growth for the second straight month, -1.31% vs. -3.40% respectively. However, the gap in year to date performance between growth and value remains very large as the Russell 1000 Growth is up 20.11% while the Russell 1000 Value is down -12.74% year to date.
International equity markets were also mixed with developed equities finishing October down -3.99%. Emerging market equities fared much better up +2.06% as they were lifted by strong data out of China, whose economy looks to be the only one on pace for positive GDP growth this year relative to 2019. Year to date, emerging market equities have also outpaced their international developed counterparts, +0.87% vs. -10.80% respectively.
In the fixed income markets, we saw both taxable and municipal bonds fall during October, despite the less than stellar returns from the equity markets. In any given month, about 60% of the time stocks and bonds tend to move in opposite directions; another 20% of the time they tend to both go up, and this October was one of the remaining 20% when they both moved lower. The Bloomberg Barclays US Aggregate Bond Index finished the month down -0.45% while the Barclays Municipal Index was down -0.30%. Treasury yields moved higher throughout the month with the 10-year yield rising by 18 basis points to 0.88%. This resulted in a negative return in the treasury market of -0.94% for the month. The foreign bond market showed stronger returns in October and finished the month up +0.46% as investors moved towards safe haven assets overseas. High yield fixed income benefited from a strong start to the month when equities were up, finishing the month +0.47% higher.
Throughout October, markets weighed the virus and election developments. The virus continued trending up and Europe is, unfortunately, suffering a serious second wave. With European governments implementing new restrictive measures, high frequency data in Europe has begun trending lower. Recent data has shown some recovery in the manufacturing sector while the service sector has felt the brunt of the restrictions. Economies will begin to be increasingly impacted if these restrictions continue.
US Election
While the United States also has its Covid challenges, Americans were focused on the election as well. In early October, as the polls widened in favor of a Democratic Blue Wave, the market balanced the increasing likelihood of a substantial stimulus bill and the decreasing chance of a contested election with the less favorable business environment that would result. At the end of the month, the markets pulled back due to the consensus prediction that the Blue Wave was not going to happen.
With our independent lens, we would sum up election day and say Americans voted for peace and quiet. As we write this, the Associated Press has called the Presidential election for Joe Biden though President Trump has yet to concede awaiting the outcome of his legal challenges. We would say the biggest losers on election night were the President, Pollsters, and Progressives.
While the election turned out to be much closer than the polls and press had reported, it appears Donald Trump has lost the Presidency. According to multiple sources, Democrats outspent the Republicans 2:1 and ultimately seven states proved to be battlegrounds with margins of victory under 3%. While the votes are still being counted in some states, and even being recounted in Georgia, it is likely Joe Biden has won the electoral college. While possible, Trumps lawsuits would need to overturn the results in several swing states to make a difference. But this is 2020 so we are not ruling anything out. It is clear the pollsters are ultimately the biggest losers as this is the second Presidential election in a row that they have gotten badly wrong. We think Progressives also lost in this election as, collectively, Americans split their ticket. While it appears that Biden won the Presidency, there were no significant swings in the US Senate and there was a decrease in the Democratic House Majority. The Democrats also lost a governorship and control of several state capital legislatures. Moreover, even in deep blue Illinois and California, ballot measures to raise taxes were rejected by the state’s voters. California also rejected a ballot measure to implement affirmative action. So collectively, we view this as Americans saying they want a calmer President, but the Progressive agenda went too far.
Interestingly, the only ballot measure to do well across the country was legalized marijuana!
Both President Donald Trump and Former Vice President Joe Biden experienced wins and losses in the election. Even though Biden successfully reversed the electoral college victory of Trump in 2016, Biden had the weakest coattails of any President since 1960 and he is likely to be the first Democratic President to take office since 1884 with the Senate in control of the other party (Mehlman).
Each party will now start the usual cycle of post-election lessons learned. What will the Republicans look like post- Trump? What will the outcome in the Democratic party be between the Progressives who will argue the party did not go far enough and the moderates who argue they went too far? Everything is changed and yet nothing has changed. America remains a country divided. Unlike most elections, 83% of voters said it matters who wins the 2020 Presidential election – they just could not agree on who the winner should be. And finally, if you are a political junkie
– you still have the two Georgia Senate runoffs in early January to keep you interested.
Vaccines
Even before the Pfizer vaccine announcement Monday, we had planned on doing a deeper dive into vaccines this month. Many of the pandemic efforts thus far have focused on buying time by slowing the spread of the virus and flattening the curve through social distancing, wearing masks etc. while a vaccine is being developed. So, let us leave politics behind and take a brief detour into the efforts to discover, develop and deploy one or more vaccines. There has been a concerted effort from the private healthcare sector to develop a Coronavirus vaccine which has been directly supported by the US government (and others).
We will start with a look at the historical process before we examine how the timeline has been accelerated. Historically the vaccine approval process has been a series of incremental steps lasting seven to ten years. This starts with the development of a promising compound, followed by a series of pre-clinical trials. These pre-clinical trials are designed to test the efficacy and safety of the drug without human testing. If the company receives favorable trial data, they can then file an application with the U.S. Food and Drug Administration (FDA) to begin clinical studies and trials. There are three main sequential phases once the company’s application has been approved by the FDA. Each one increasing in time and cost but not begun until passing the prior phase’s hurdles.
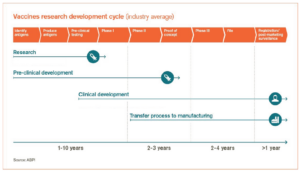
Phase 1: This trial is focused on determining the safety of the drug in question. It usually consists of 20 to 80 healthy volunteers who will be exposed to the drug and monitored for any potential side effects and impact on the body. This study will also help to determine the dosage and the most effective treatment protocol. This stage usually takes less than one year and 70% of potential new drugs pass into Phase 2.
Phase 2: This trial is focused on the effectiveness of the drug. There are hundreds of participants who have a certain disease or condition in this trial. Participants are given either the new drug or a placebo (or different drug). These two groups are then compared to determine the efficacy of the product. Safety continues to be evaluated and participants are monitored for short-term side effects. This phase can last up to two years and 33% of drugs pass into Phase 3.
Phase 3: This trial is designed to test both the effectiveness and the safety of the drug across a larger sample size (usually thousands of participants) across multiple age groups and races. Different dosages are tested, and
participants continue to be monitored for side effects. This phase typically lasts for one to three years and 25-30% of drugs will pass.
About 5% of all drugs that start the process make it through all three phases. Companies that develop drugs that do progress through these phases can submit another application to the FDA while test participants undergo ongoing monitoring for long-term side effects. The FDA reviews the results from the trials and determines whether the drug is approved and if the pharmaceutical company can begin to market the drug to the public. All in all, this process can take around 7-10 years from start to finish and require a significant amount of money.
President Trump implemented Operation Warp Speed (OWS) on May 15th with the ambitious goal of producing and delivering 300 million doses of a successful vaccine starting by January 2021, while following the FDA’s safety and effectiveness guidelines. The initiative allowed companies to significantly cut down the timeline of the vaccine approval process. How have they accelerated the timeline? They have absorbed the development costs from companies by running concurrent studies instead of sequential ones. From a development standpoint, protocols for the demonstration of safety and efficacy have been aligned under OWS which has allowed companies to combine multiple phases of drug development while receiving government support. Moreover, the government funding has allowed for the manufacturing of promising vaccine candidates at an industrial scale well before demonstration of efficacy and safety. While increasing the financial risk, this does not impact the product risk and allows for vaccine delivery as soon as the candidate receives FDA approval. If the vaccine wins approval, the manufacturing side already has a head start. If the vaccine fails to progress, the samples are unused, and the government absorbs the cost of the accelerated production.
The cost to develop a vaccine fully can, of course, vary but it has been estimated to be $500 million to $2 billion dollars. This can obviously be cost prohibitive for many companies, but the government has taken unprecedented steps to financially support companies which are looking to develop a Coronavirus vaccine. As President Trump has noted, they are focused on having as many “shots on net” as possible. The US government has flooded nearly $10 billion into vaccine production and manufacturing to this point and will likely spend more if needed.
While there are several vaccines under different stages of development, here are the top candidates now:
Moderna: The company began development of their vaccine in January 2020 and has received over $1 billion in government support since that time. They started human trials in March and have progressed into Phase 3 trials which began on July 27th. Under the FDA guidelines, they recruited 30,000 participants for their Phase 3 study, which has a diverse participant base. Their trial is well underway, but the company will have to wait until the sample of sick patients becomes significant enough to determine efficacy before moving forward. If the results meet FDA benchmarks (at least 50% effectiveness) then they could seek emergency use authorization by the end of 2020. In August, the company received another $1.5 billion in government funding in exchange for 100 million doses.
Pfizer: This vaccine is a partnership with German firm BioNTech and is designed to be given in two doses. Phase 1/2 was launched in May of this year with two different versions of the vaccine. One of these moved on into Phase 2/3 trials on July 27th, and quickly gathered 30,000 volunteers across multiple countries. After strong initial results from the first dose, they expanded the trial to 43,000 participants in September, and gained approval to start testing the vaccine on children as young as 12 in October. On November 9th, they announced 90% success with their vaccine. The US government awarded a $1.9 billion contract to produce 100 million doses by December (with the option to acquire 500 million more doses). They have also made deals with Japan and the EU. Pfizer expects to manufacture over 1.3 billion doses worldwide by the end of 2021.
Johnson & Johnson: The company received $456 million from the US government to support the development and production of their vaccine in March. They began Phase 1/2 trials in July and launched Phase 3 trials with 60,000 participants in September. The vaccine only requires one dose, unlike the other vaccines in Phase 3 trials. Despite the trial being paused in October to address a reaction from a volunteer, the company still expects to get results by the end of the year. The US government has paid $1 billion for 100 million doses if the vaccine is approved, and they have entered into a similar agreement with the European Union.
AstraZeneca: In collaboration with the University of Oxford, the company has developed a vaccine that showed strong results in pre-clinical trials. They were awarded $1.2 billion from the US government in May of this year to produce 300 million doses of the vaccine. They passed through Phase 1/2 trials and moved onto Phase 3 trials globally in August. They have had their Phase 3 study paused a couple of times due to possible side effects seen in volunteers, but these have been cleared up and the trial has pushed on.
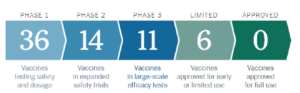
While these companies hold the top vaccine candidates, there are several candidates across the globe that are under development. There are 11 vaccines that are in Phase 3 trials and are showing promise. In addition to the four listed above, there has been success from a few companies overseas including one vaccine in Russia, four in China, one in India, and one in Australia. While these vaccines are in Phase 3 studies as well, they would still have to go through FDA approval in the US, which has much higher standards than these other countries. Therefore, it is less likely that these would be approved in the US.
Once we do have a vaccine approved, OWS has been successful in creating a supply of doses that will be readily available on day one. This is important as it has been indicated that 50-60% of the US population will need to receive the vaccination for the population to be considered at less risk. The other question will be around the programs to administer the vaccine. The Trump administration has noted that they have the military on standby to help with distribution, but the states have indicated that they will need additional funding from the government to administer the vaccine (upwards of $8 billion).
Coming up with the vaccine is only a part of the challenge. Other questions for each vaccine include:
- How effective it is at either preventing the disease or reducing its impact?
- How many doses are required?
- How long will its protection last?
- Is it for 6-12 months as in the common flu vaccine?
- A decade like the Mumps vaccine?
- Longer than two decades like Rubella and Hepatitis B vaccines?
- Are there any special requirements for storing or shipping the vaccine such as super cold refrigerators?
As in any great scientific endeavor there will be many setbacks and “failures” along the way, but we are loath to bet against the entrepreneurial motivations of the world’s scientists.
Year-End Financial Planning
As the Procyon Investment Committee looks ahead, we are always monitoring macro trends, individual companies, elections, and Covid status and treatments. Regardless of which politicians are in power in January 2021, there will continue to be strategic financial planning opportunities for clients. In our final section we will review some actions one might consider before year end.
With a big sigh of relief, we do not feel any extraordinary estate tax planning is necessary prior to year-end.
While there is speculation around how long the current, historically low tax rates will continue, there is no doubt that the IRS will claim its due on tax-deferred accounts like IRAs. If you are anticipating having a higher income tax bracket in the future because of either changing personal circumstances or rising rates, the following are some potentially relevant strategies.
A full or partial ROTH Conversion merits consideration. A ROTH Conversion is the taxable transfer from a Traditional IRA to a ROTH IRA. While the account owner pays income taxes from taxable assets on the amount withdrawn from the Traditional IRA, the owner benefits in three ways through the ROTH Conversion. First, the funds in the ROTH grow tax free. Second, there are no lifetime RMDs from a ROTH. Third, there will be fewer funds remaining in the Traditional IRA subject to RMDs and taxes.
Voluntary Traditional IRA withdrawals may be relevant for those between 59.5 and 72 years of age. The goal is to solve for the lowest lifetime income tax paid by pulling forward a portion of the IRA distributions into earlier years and avoiding a larger RMD in later years that can spike one into higher tax brackets. In addition, since the SECURE Act eliminated stretch IRAs for most non-spouse beneficiaries, if the extra cash is not needed one could leverage cash value life insurance as an alternative estate planning strategy. The life insurance cash value grows tax-free during the owner’s life; upon death, the policy’s proceeds are passed to the beneficiary income and estate tax-free.
If you are charitably inclined, there are some additional year end strategies to consider. The CARES Act made available to all taxpayers a $300 deduction for charitable contributions.
Though the CARES Act also waived Required Minimum Distributions (RMDs) in 2020, some clients are still using their Traditional IRAs to make charitable contributions via Qualified Charitable Distributions (QCDs). These are direct transfers of IRA funds to a qualifying charity. IRA owners who are at least 70 ½ can make QCDs up to $100,000 per year, regardless of whether they claim the standard deduction or itemize. This technique may be appealing if you have few other deductions or are close to your charitable deduction limits and are seeking efficient ways to decrease IRA funds at zero tax cost.
There are many ways to donate to a qualified charity in tax-efficient ways. From gifting highly appreciated stock to setting up a Donor Advised Fund to establishing Charitable Remainder Trusts or Charitable Gift Annuities, our Procyon Team is available to listen to what is important to you and provide perspective on the right solution for your situation.
Lastly, and as always, Procyon has proactively taken the opportunity throughout the course of 2020 to do tax loss selling to minimize capital gains exposure.
We hope you found this helpful. All of us at Procyon are cognizant of the difficult time we have navigated this year. Many individuals, families and communities have been affected by the Covid virus in a way that will leave their lives forever changed. We hope with the development of effective treatments and vaccines that those difficulties will slow and eventually stop for all of us.
All the best.
Download PDF BACK